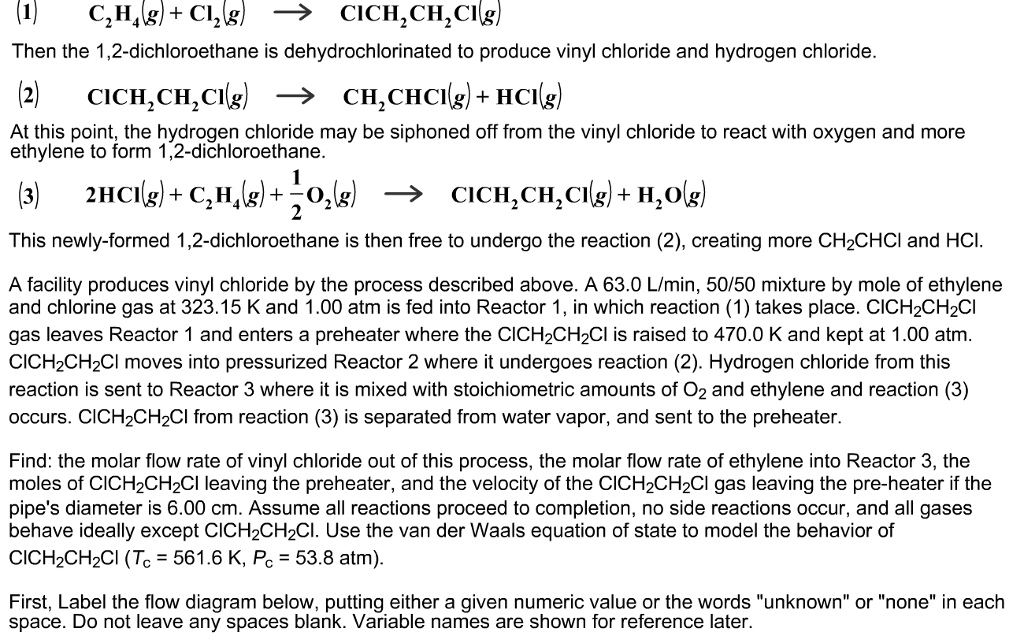
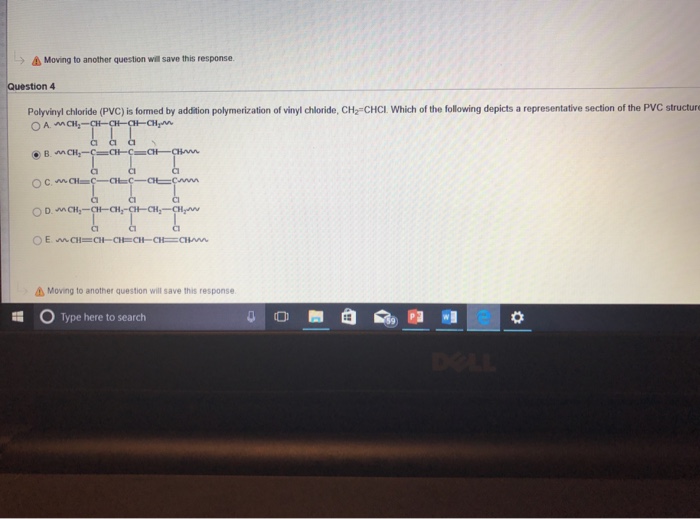
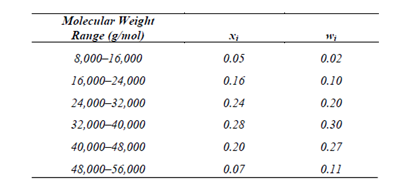
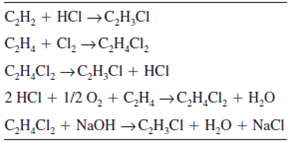Write The Full Structural Formula Of Vinyl Chloride
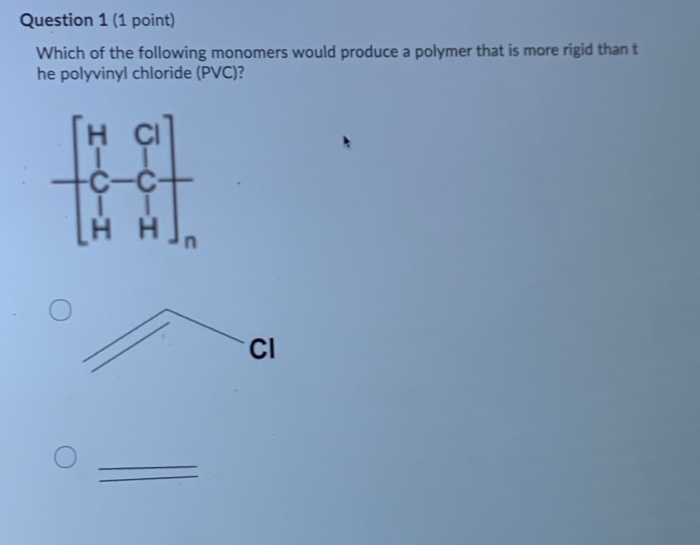
Rigid sometimes abbreviated as rpvc and flexible.
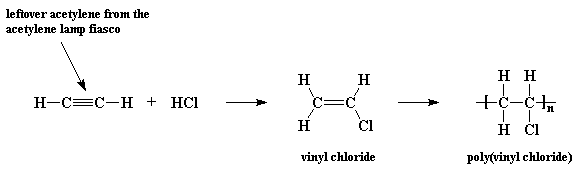
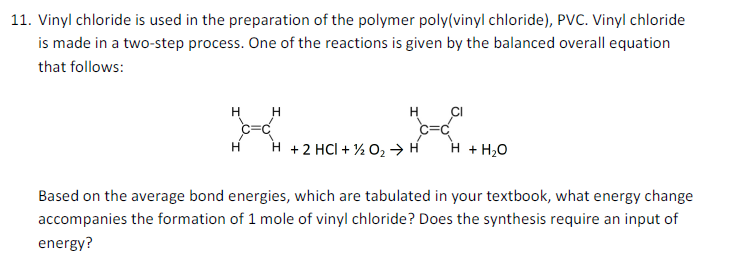
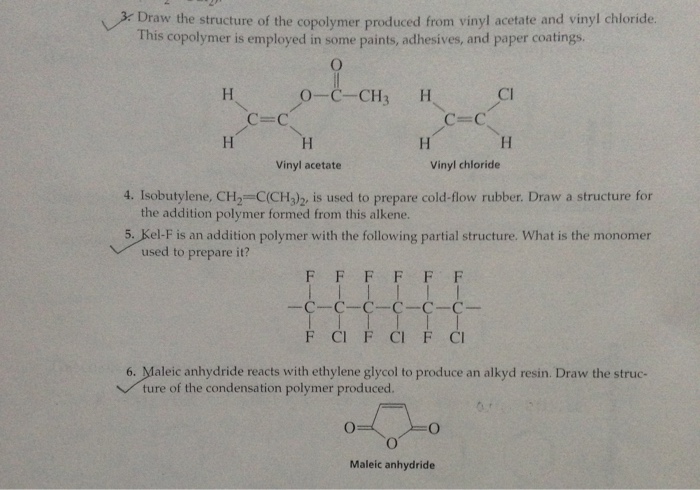
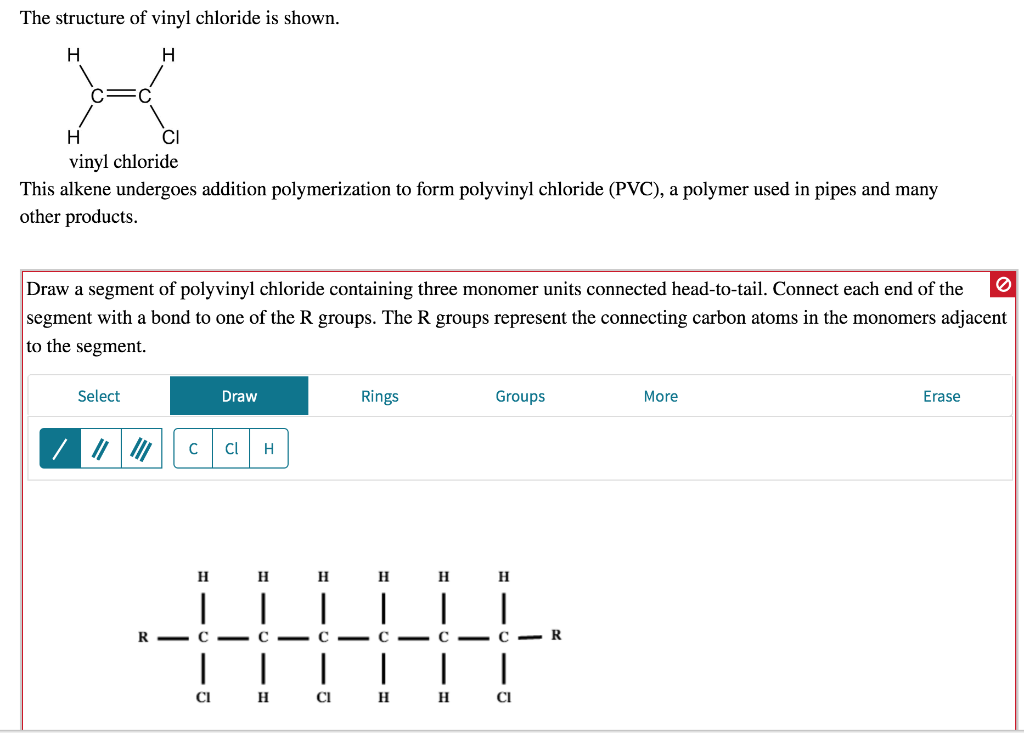
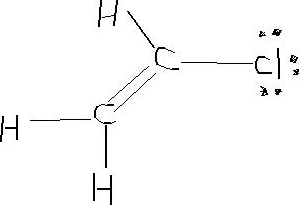
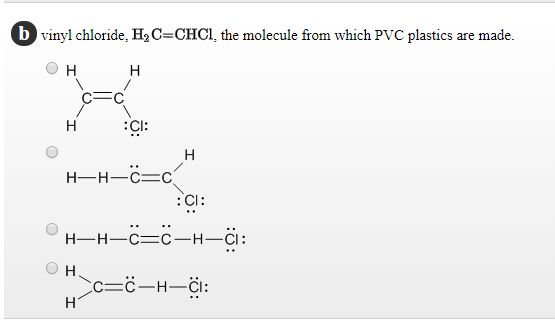
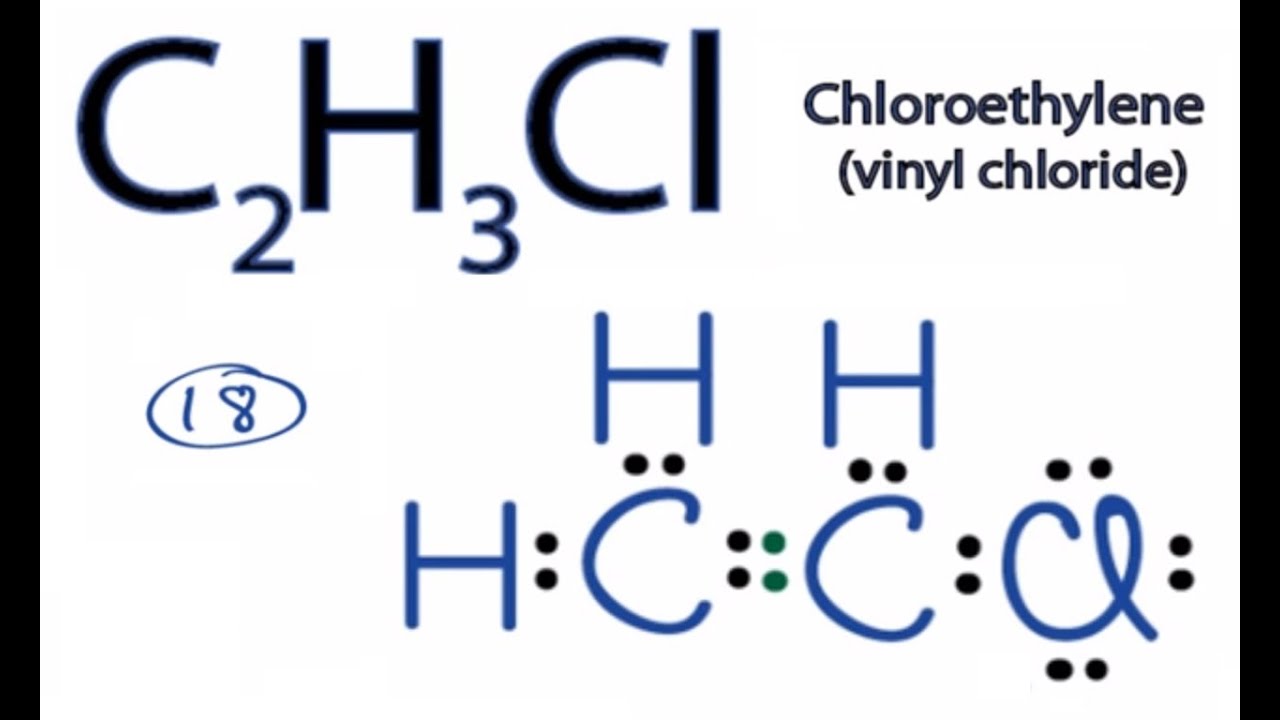
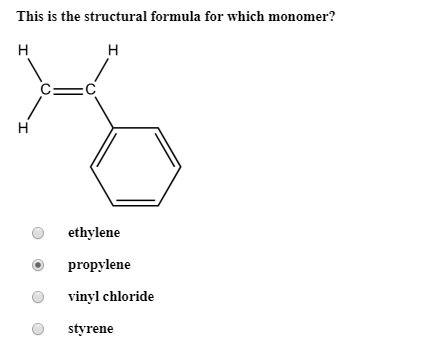
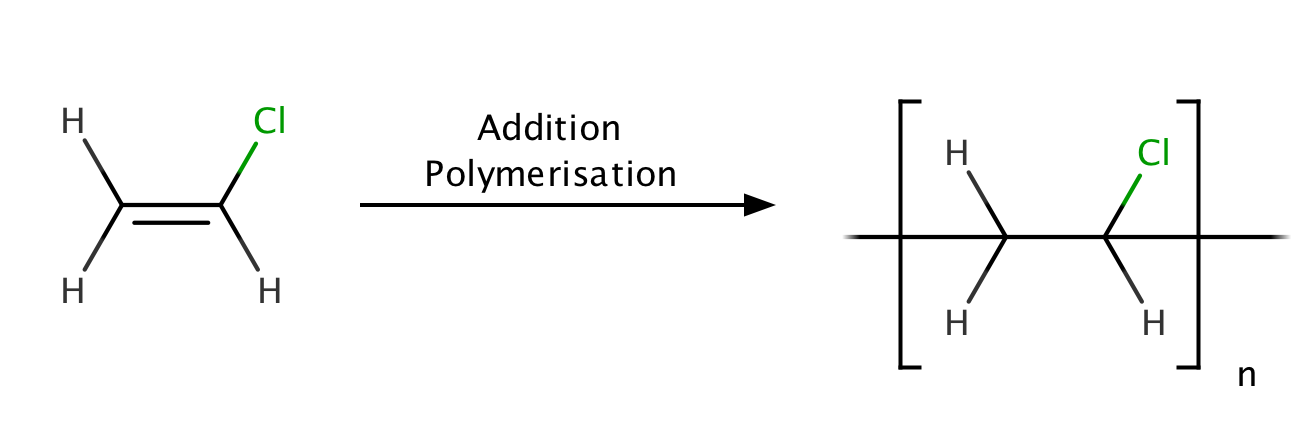
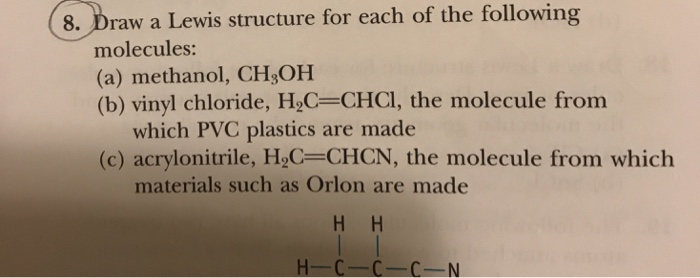
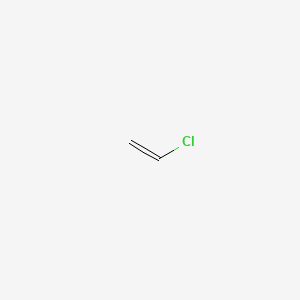
Write the full structural formula of vinyl chloride. 2 3 specify the type of the bond between the two carbon atoms. Vinyl chloride ch 2 chcl also known as chloroethylene is most often obtained by reacting ethylene with oxygen and hydrogen chloride over a copper catalyst. Balance the formula equation by placing coefficients numbers in front of formulas so that you have the same total number of each kind of atom on both the reactant side and the product side. It is a clear colorless liquid with a sweet fruity smell.
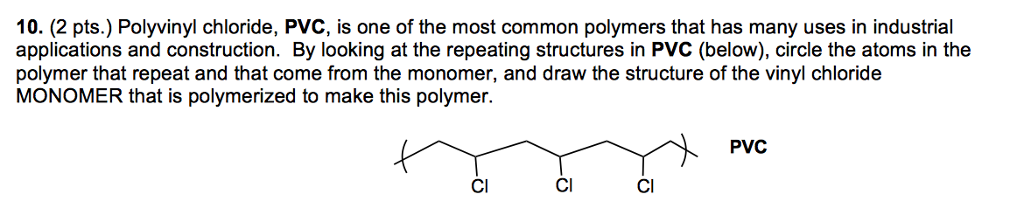
Show at least four repeat units. It is a toxic and carcinogenic gas that is handled under special protective procedures. Starting material for the preparation of pvc plastics b c hbrcif halothane. Vinyl chloride h2c chcl or c2h3cl n or c2h3cl cid 6338 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more.
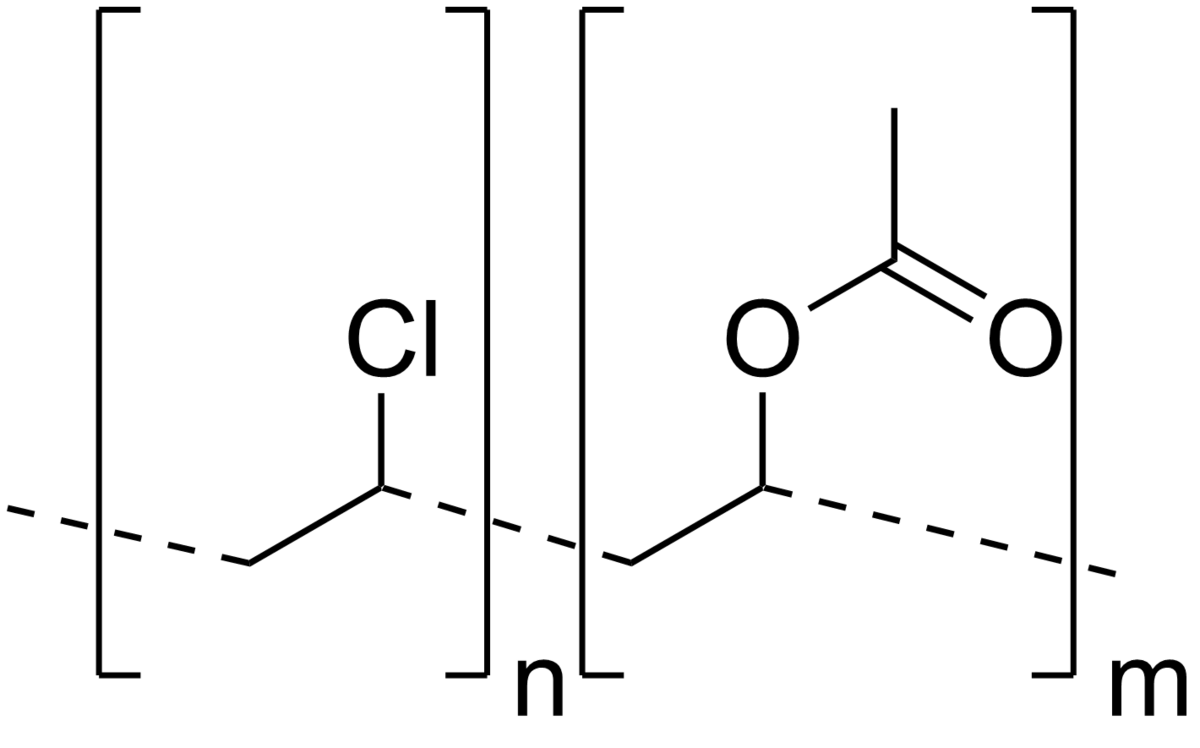
What is the repeat unit in polyvinylidene chloride. 3 1 give the condensed structural formula of. All three fluorines are bonded to the same carbon c cci f4 freon 114. A nonflammable inhalation anesthetic.
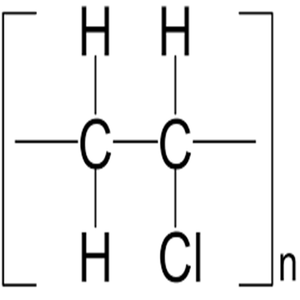
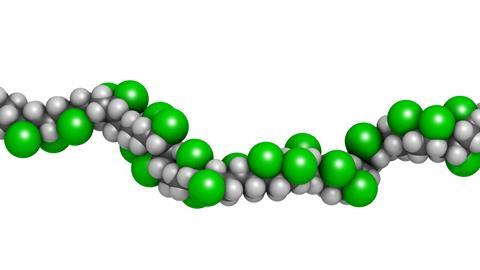
The structural formula is. 2 2 give the molecular formula of this compound. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride pvc. Pvc is the world s third most widely produced synthetic plastic polymer after polyethylene and polypropylene about 40 million tons of pvc are produced each year.
This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride pvc. Each carbon bears one. Vinyl acetate is an industrial chemical that is produced in large amounts in the united states. Remember that in chemical reactions atoms are just rearranged not created or destroyed.
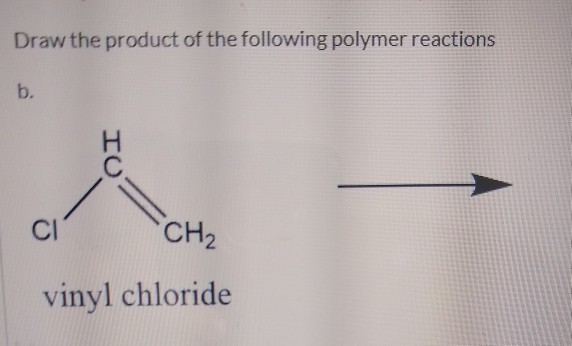
Vinyl chloride is an organochloride with the formula h 2 c chcl that is also called vinyl chloride monomer vcm or chloroethene. Write the correct formula for each reactant and each product. Write the condensed structural formula for the polymer formed from di methyl sialon. About 13 billion kilograms are produced annually.
2 1 write the structural formula of vinyl chloride. A ch ci vinyl chloride. Vinyl acetate is used to make other industrial chemicals. Formerly used as a refrigerant and as an aerosol propellant.
Pvc comes in two basic forms. A segment of the polymer is represented as write the structure of the polymer made from vinyl fluoride ch 2 chf. Vinyl chloride is an organochloride with the formula h2c chcl that is also called vinyl chloride monomer vcm or chloroethene.